SANOFI DELIVERS BUSINESS EPS GROWTH OF 7.3% AT CER IN 2014
Feb 5, 2015
PARIS, Feb. 5, 2015 /PRNewswire/ -- Sanofi (NYSE: SNY; EURONEXT: SAN)
|
Q4 2014 |
Change |
Change |
2014 |
Change |
Change |
|
|
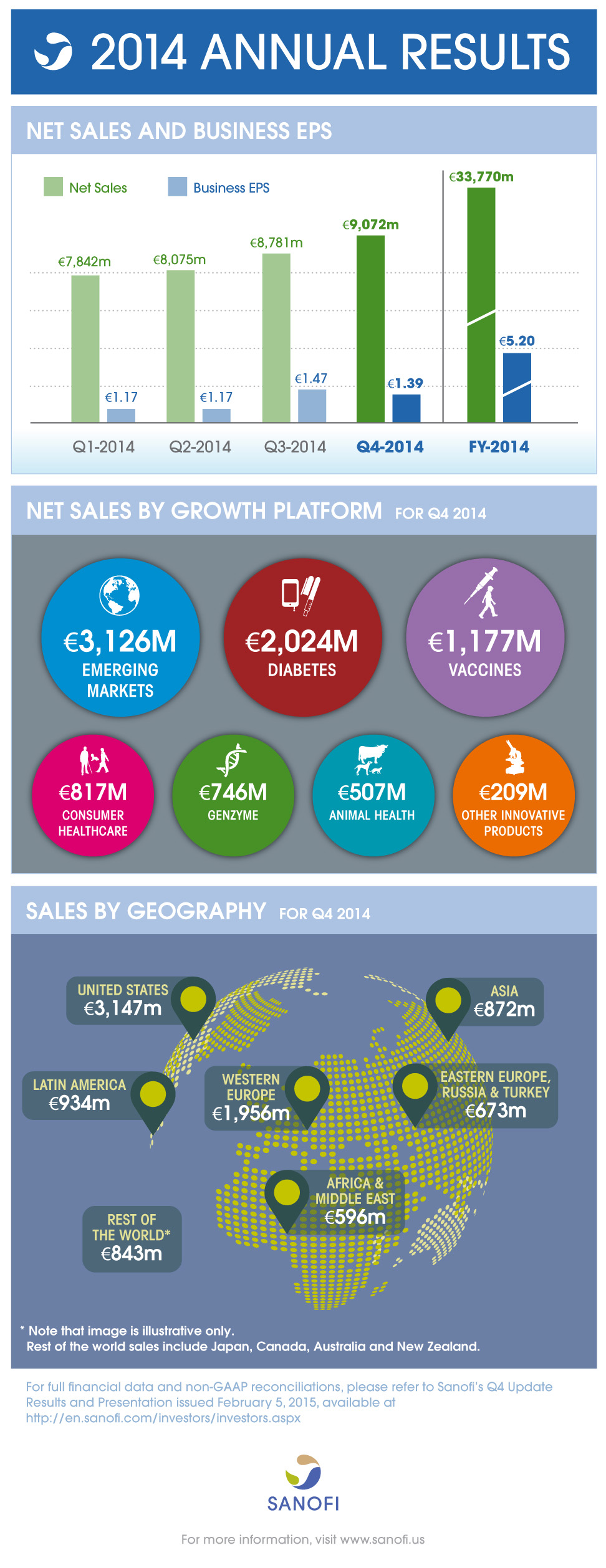
Net sales |
€9,072 m |
+7.3% |
+4.6% |
€33,770 m |
+2.5% |
+4.9% |
|
Business net income(1) |
€1,828 m |
+0.8% |
-0.3% |
€6,847 m |
+2.4% |
+6.7% |
|
Business EPS(2) |
€1.39 |
+1.5% |
- |
€5.20 |
+3.0% |
+7.3% |
In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income(1) is a non-GAAP financial measure. (2)(EPS) Earnings Per Share
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7426551-sanofi-results-q4-2014/

Commenting on the Group's performance in Q4 2014, Sanofi Chairman and Chief Executive Officer, Serge Weinberg said, "We are pleased with our solid performance in 2014. The Group delivered strong financial results and we made significant progress in bringing new medicines to market. In 2015, our focus is on operational excellence as we launch multiple new medicines and vaccines. We will also invest in our R&D projects to maximize their potential. The combination of our innovative pipeline and sustainable, diversified businesses provides a strong foundation to create long-term shareholder value."
Q4 2014 and Annual Performance
- In the fourth quarter of 2014, Sanofi generated sales of €9,072 million, an increase of 7.3% on a reported basis. Exchange rate movements had a positive effect of 2.7 percentage points mainly reflecting the strength of the dollar. Full-year sales reached €33,770 million, an increase of 2.5% on a reported basis. Exchange rate movements had an unfavorable effect of 2.4 percentage points reflecting the strength of the euro versus other currencies, in particular the Japanese Yen, Russian Ruble, Brazilian Real and Argentine Peso.
- In the fourth quarter, sales of the Diabetes division increased 11.0% to €2,024 million driven by double-digit growth of Lantus® in the U.S., Emerging Markets and Western Europe. Full-year sales of the Diabetes division grew 12.1% to €7,273 million.
- Sales of Consumer Healthcare products (CHC) were €817 million in the fourth quarter, an increase of 14.0%. Several products (amounting to €68 million in sales) previously recorded in prescription pharmaceuticals in the fourth quarter of 2013 were transferred to Consumer Healthcare products. Excluding this category change, sales of CHC grew 4.2% driven by the success of the Nasacort® Rx-to-OTC switch in the U.S. and good performance of Doliprane®and Enterogermina®. Sales of Nasacort® Allergy 24HR nasal spray, which has been available over-the-counter (OTC) in the U.S. since February 2014, were €17 million in the fourth quarter in the U.S. In 2014, sales of CHC were €3,337 million, an increase of 16.5%. Excluding the category change mentioned above (€273 million in 2013), CHC sales grew 6.8%.
- Fourth-quarter sales of Genzyme grew 22.2% to €746 million boosted by the performance of Aubagio®. Sales of Genzyme grew 28.6% to €299 million in the U.S., 16.3% to €229 million in Western Europe, 22.3% to €154 million in Emerging Markets and 17.9% to €64 million in the rest of the World. In 2014, sales of Genzyme reached €2,604 million, an increase of 24.3%.
- Fourth-quarter sales of Other Innovative Products were €209 million, an increase of 5.9% in the fourth quarter and €815 million, an increase of 14.7% in 2014.
- Sales of Generics were down 0.6% to €467 million in the fourth quarter, reflecting lower sales in Western Europe(down 7.7% to €133 million) and the U.S. (down 25.7% to €29 million) which offset the recovery in Brazil (up 22% to €71 million ). Full-year sales of Generics grew 16.2% to €1,805 million (down 2.8% excluding Brazil generics).
- In the fourth quarter, consolidated sales of Sanofi Pasteur increased 16.2% to €1,177 million reflecting strong performance of influenza vaccines in the U.S. and Emerging Markets as well as the continued recovery of Pentacel® in the U.S. Fourth-quarter sales increased 22.3% (to €653 million) and 19.9% (to €441 million) in the U.S. and Emerging Markets, respectively. Full-year consolidated sales of Sanofi Pasteur grew 7.2% to €3,974 million, despite remaining capacity constraints (which Sanofi continues to address in 2015) in a context of strong demand.
- Fourth-quarter sales of Animal Health increased 11.5% to €507 million sustained by the success of the NexGard™launch and performance of fipronil products after a difficult 2013. In the U.S., Animal Health sales increased 35.7% to €170 million in the fourth quarter. Full-year sales of Animal Health increased 6.7% to €2,076 million.
R&D Update
Regulatory updates since the publication of the third-quarter 2014 results on October 28, 2014 include the following:
- In January, the European Medicines Agency (EMA) has accepted for review the Marketing Authorization Application (MAA) for Praluent™ (alirocumab, in collaboration with Regeneron). In January, the U.S. Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for Praluent™. Praluent™is an investigational monoclonal antibody targeting PCSK9 that is intended for the treatment of patients with hypercholesterolemia.
- In January, the rolling submission for Dengue vaccine was initiated in several endemic countries in Asia.
- In January, the European Commission approved NexGard® Spectra™ (afoxolaner and milbemycin oxime), a soft, beef-flavored chew for dogs that provides a broad spectrum of internal and external parasite control in one monthly dose.
- In January, the EMA has accepted for review the Marketing Authorization Application (MAA) for the pediatric hexavalent vaccine, PR5i.
- In January, the European Commission approved Cerdelga® (eliglustat), an oral treatment for certain adults living with Gaucher disease type 1.
- In December, Fluzone® Quadrivalent ID vaccine (a four-strain influenza vaccine which uses a microinjection system for intradermal delivery) was approved in the U.S.
- In November, the FDA approved the supplemental biologics license application (sBLA) for Fluzone® High-Dose(Influenza Vaccine) to include efficacy data in the Prescribing Information. These data demonstrate that Fluzone®High-Dose vaccine provided improved protection against influenza compared to standard-dose Fluzone® vaccine (trivalent intramuscular formulation) in adults 65 years of age and older.
- In November, the FDA granted Breakthrough Therapy designation for dupilumab, an investigational monoclonal antibody that blocks IL-4 and IL-13, two cytokines required for the Th2 immune response, for the treatment of adults with moderate-to-severe atopic dermatitis that are not adequately controlled with topical prescription therapy and/or for whom these treatments are not appropriate.
- In November, the FDA approved Lemtrada® (alemtuzumab) for the treatment of patients with relapsing forms of multiple sclerosis and who have had an inadequate response to two or more drugs.
- At the beginning of February 2015, the R&D pipeline contained 43 projects (excluding Life Cycle Management) and vaccine candidates in clinical development of which 14 are in Phase III or have been submitted to the regulatory authorities for approval.
2015 Guidance
- Taking into account the outlook for U.S. Diabetes as well as new product launches and late stage pipeline development, 2015 Business EPS(1) is expected to be stable to slightly growing versus 2014 at constant average exchange rates, barring major unforeseen adverse events(5)
- Applying December 31, 2014 exchange rates to this full-year 2015 guidance, the additional positive currency impact on 2015 business EPS is estimated to be between 4% and 5%.
To access the full press release of the Q4 2014 results, please click here.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group's ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment policies and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2013. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
About Sanofi
Sanofi, a global and diversified healthcare leader, discovers, develops and distributes therapeutic solutions focused on patients' needs. Sanofi has core strengths in the field of healthcare with seven growth platforms: diabetes solutions, human vaccines, innovative drugs, rare diseases, consumer healthcare, emerging markets, animal health and the new Genzyme. Sanofi is listed in Paris (EURONEXT: SAN) and in New York (NYSE: SNY).
Sanofi is the holding company of a consolidated group of subsidiaries and operates in the United States as Sanofi US. For more information on Sanofi US, please visit http://www.sanofi.us or call 1-800-981-2491.
Sanofi US is also on Twitter and Facebook. Visit us at https://twitter.com/SanofiUS andhttp://www.facebook.com/sanofiUS.
Media Relations:
908-989-0726
Email: [email protected]
Investor Relations:
908-981-5560
E-mail: [email protected]